NarcoBond®© TM: A Novel Drug Overdose Decoy Receptor Countermeasure
The Problem: the opioid epidemic is causing an unprecedented number of deaths, surging to over 100,000 per year in the United States. Adding to this carnage, a wave of overdose deaths is being wrought by a deadly cocktail of fentanyl and xylazine. Xylazine, also known as “Tranq” or “zombie drug”, is a powerful sedative ap-proved only for veterinary use and is incredibly dangerous to people on its own. This surge is so concerning that the Biden administration declared the fentanyl-xylazine drug cocktail a national threat. Xylazine increases the profits of traffickers by extending the high or euphoria of opioids, affording a reduction in the amount of pre-cious opioid needed. Since xylazine is not an opioid, naloxone (Narcan) does not reverse its effect and no anti-dote for xylazine is approved for human use. Xylazine-fentanyl cocktails have a high potential for fatal overdose since both substances depress respiratory function and this interaction appears synergistic. Drug cartels are increasingly adding xylazine to illicit fentanyl, with a quarter of overdoses now linked to opioid laced with xylazine.
Our Solution: NarcoBondα2/µO – a “nanosponge” containing decoy receptors for fentanyl (mu opioid receptor) and xylazine (α2A-adrenergic receptor -α2AAR) safely corrals the drugs in the peripheral blood. As a result, the drugs are trapped away the central nervous system, thus sparing the patient from the high CNS concentrations that depress respiration and cause death. Previously, we used our platform to develop nanasponges for opioids and methamphetamines. Crucially, we showed life-saving effects in rodents with NarcoBondµO, a nanosponge that sequesters opioids in the peripheral blood from where the opioids are safely cleared.
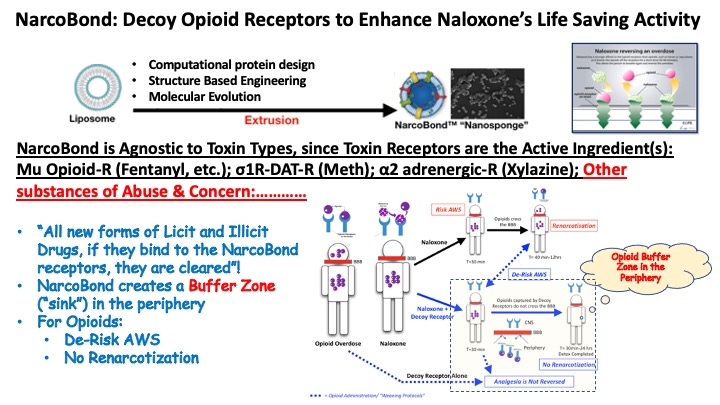
“NarcoBond” nanospheres are assembled from a mixture of cholesterol & synthetic choline-based phos-pholipids that mimic a cell membrane. Its multifunctional surface displays these human membrane proteins in their natural environment which optimizes the binding function of decoyed receptors and the capture of drugs. As a 100 nm nanosponge, NarcoBond resides in the peripheral circulation and thus safely sequesters over-dosed drugs away from the CNS. This results in an antagonistic pharmacological effect by rapidly reducing free plasma and CNS concentration of the circulating drug(s). With time, drugs are then safely cleared by standard routes of elimination. NarcoBond is agnostic to toxin types, since toxin receptors are the active Ingredient(s):Mu Opioid-R (Fentanyl, etc.); σ1R-DAT-R (Meth); α2 adrenergic-R (Xylazine); Other substances of Abuse & Concern:…………
** NarcoBond is a registered trademark of CiBots, Inc.
References
- DEA Reports Widespread Threat of Fentanyl Mixed with Xylazine | DEA.gov https://www.dea.gov/alert/dea-reports-widespread-threat-fentanyl-mixed-xylazine.
- Yaksh, T.L. (1985). Pharmacology of spinal adrenergic systems which modulate spinal nociceptive processing. Pharmacol. Biochem. Behav. 22, 845–858. 10.1016/0091-3057(85)90537-4.
- De Andres, J., Hayek, S., Perruchoud, C., Lawrence, M.M., Reina, M.A., De Andres-Serrano, C., Rubio-Haro, R., Hunt, M., and Yaksh, T.L. (2022). Intrathecal Drug Delivery: Advances and Applications in the Management of Chronic Pain Patient. Front. Pain Res. 3.
- Akeju, O., and Brown, E.N. (2017). Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr. Opin. Neurobiol. 44, 178–185. 10.1016/j.conb.2017.04.011.
- Flükiger, J., Hollinger, A., Speich, B., Meier, V., Tontsch, J., Zehnder, T., and Siegemund, M. (2018). Dexmedetomidine in prevention and treatment of postoperative and intensive care unit delirium: a systematic review and meta-analysis. Ann. Intensive Care 8, 92. 10.1186/s13613-018-0437-z.
- Hemmings, H.C., Riegelhaupt, P.M., Kelz, M.B., Solt, K., Eckenhoff, R.G., Orser, B.A., and Goldstein, P.A. (2019). Towards a Comprehensive Understanding of Anesthetic Mechanisms of Action: A Decade of Discovery. Trends Pharmacol. Sci. 40, 464–481. 10.1016/j.tips.2019.05.001.
- Ballesteros, J.J., Briscoe, J.B., and Ishizawa, Y. (2020). Neural signatures of α2-Adrenergic agonist-induced unconsciousness and awakening by antagonist. eLife 9, e57670. 10.7554/eLife.57670.
- Clarke, S.F.J., Dargan, P.I., and Jones, A.L. (2005). Naloxone in opioid poisoning: walking the tightrope. Emerg. Med. J. 22, 612–616. 10.1136/emj.2003.009613.
- Philipp, M., Brede, M., and Hein, L. (2002). Physiological significance of α2-adrenergic receptor subtype diversity: one receptor is not enough. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 283, R287–R295. 10.1152/ajpregu.00123.2002.
- Cornil, C.A., and Ball, G.F. (2008). Interplay among catecholamine systems: Dopamine binds to α2-adrenergic receptors in birds and mammals. J. Comp. Neurol. 511, 610–627. 10.1002/cne.21861.
- Kacinko, S.L., Mohr, A.L.A., Logan, B.K., and Barbieri, E.J. (2022). Xylazine: Pharmacology Review and Prevalence and Drug Combinations in Forensic Toxicology Casework. J. Anal. Toxicol. 46, 911–917. 10.1093/jat/bkac049.
- Johnson, J., Pizzicato, L., Johnson, C., and Viner, K. (2021). Increasing presence of xylazine in heroin and/or fentanyl deaths, Philadelphia, Pennsylvania, 2010–2019. Inj. Prev. 27, 395–398. 10.1136/injuryprev-2020-043968.
- George, S., Boulay, S., and Begley, D. (2010). “I saved a life”: a heroin addict’s reflections on managing an overdose using “take home naloxone.” BMJ Case Rep. 2010. 10.1136/bcr.05.2010.2986.
- Le Bars, D., Gozariu, M., and Cadden, S.W. (2001). Animal models of nociception. Pharmacol. Rev. 53, 597–652.
- Yoo, J.-W., Irvine, D.J., Discher, D.E., and Mitragotri, S. (2011). Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat. Rev. Drug Discov. 10, 521–535. 10.1038/nrd3499.
- Molinaro, R., Corbo, C., Martinez, J.O., Taraballi, F., Evangelopoulos, M., Minardi, S., Yazdi, I.K., Zhao, P., De Rosa, E., Sherman, M.B., et al. (2016). Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat. Mater. 15, 1037–1046. 10.1038/nmat4644.
- Blanco, E., Shen, H., and Ferrari, M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951. 10.1038/nbt.3330.
- Bulbake, U., Doppalapudi, S., Kommineni, N., and Khan, W. (2017). Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 9. 10.3390/pharmaceutics9020012.
- Duflou, J., Darke, S., and Easson, J. (2009). Morphine Concentrations in Stomach Contents of Intravenous Opioid Overdose Deaths. J. Forensic Sci. 54, 1181–1184. 10.1111/j.1556-4029.2009.01123.x.
- Structure-guided development of selective M3 muscarinic acetylcholine receptor antagonists | PNAS https://www.pnas.org/doi/abs/10.1073/pnas.1813988115.
- Xu, J., Hu, Y., Kaindl, J., Risel, P., Hübner, H., Maeda, S., Niu, X., Li, H., Gmeiner, P., Jin, C., et al. (2019). Conformational Complexity and Dynamics in a Muscarinic Receptor Revealed by NMR Spectroscopy. Mol. Cell 75, 53-65.e7. 10.1016/j.molcel.2019.04.028.
- Schwartz, D. d., and Clark, T. p. (1998). Affinity of detomidine, medetomidine and xylazine for alpha-2 adrenergic receptor subtypes. J. Vet. Pharmacol. Ther. 21, 107–111. 10.1046/j.1365-2885.1998.00113.x.
- Allen, J.W., and Yaksh, T.L. (2004). Assessment of Acute Thermal Nociception in Laboratory Animals. In Pain Research: Methods and Protocols Methods in Molecular Medicine., Z. D. Luo, ed. (Humana Press), pp. 11–23. 10.1385/1-59259-770-X:139.

